Novel HDACi CS014
CS014 is a new chemical entity, designed as a HDAC inhibitor with a multi-modal mechanism of action. By acting as an epigenetic modulator, CS014 could target the underlying pathophysiology of several rare cardiovascular and pulmonary diseases with significant unmet medical needs. The drug has successfully concluded a Phase I trial.
CS014 has the potential to reverse the fibrosis developing in IPF as shown in preclinical models
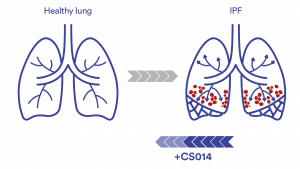
IPF and all interstitial lung diseases (ILDs) cause scarring (fibrosis) in and around the lungs' air sacs (alveoli) and airways. The lung interstitium, the space between the air sacs and the small blood vessels, contains connective tissue that plays a vital role in gas exchange. When you breathe, oxygen passes through the alveoli and interstitium into the blood, while carbon dioxide moves in the opposite direction to be exhaled. When fibrosis (red dots) develops, the lungs become stiff and lose their ability to transfer oxygen efficiently, making breathing increasingly difficult. CS014 has potential to stop or reverse the disease progression.
Mechanism of action
Mechanism of action and disease-modifying potential
CS014 employs a novel mechanism of action through epigenetic modulation, making it highly relevant for a variety of conditions, including idiopathic pulmonary fibrosis (IPF) and pulmonary arterial hypertension (PAH). In preclinical studies, CS014 has demonstrated the ability to reverse fibrosis and exhibit a dose-dependent beneficial effect on pulmonary pathological vascular remodeling, with a reduction in plexiform lesions, suggesting strong disease-modifying potential.
A therapy that directly targets thrombosis, which no currently approved or investigational treatment does, could be particularly valuable in diseases such as idiopathic pulmonary fibrosis (IPF) and pulmonary arterial hypertension (PAH), where vascular injury, abnormal clotting, and impaired blood flow are key drivers of disease progression.
In IPF, microvascular thrombosis exacerbates tissue remodeling and fibrosis. In PAH, thrombosis in the small pulmonary arteries contributes to elevated pulmonary pressure and right heart failure. By addressing the thrombotic component of these diseases, CS014 may slow disease progression, improve oxygenation, and enhance overall cardiopulmonary function.
Importantly, this mechanism of action may also have therapeutic relevance across a broader spectrum of cardiovascular and pulmonary diseases where thrombosis and vascular dysfunction play a central role.
Potential
Potential for treating rare cardiovascular and pulmonary diseases
Given its multi-modal mechanism of action, CS014 has the potential to address a broad range of cardiovascular and pulmonary diseases that currently lack effective disease-modifying therapies. The drug's ability to target fibrosis, vascular remodeling, and thrombosis positions it as a strong candidate for treating rare and life-threatening cardiovascular and pulmonary diseases.
Phase I trial
Phase I trial: Safety and tolerability
An open-label Phase I trial was successfully concluded in April 2025. The Phase I trial evaluated safety, tolerability, pharmacokinetics (PK), and pharmacodynamics (PD) of single and multiple ascending oral doses of CS014 in healthy volunteers. The trial was conducted in two parts: part one explored safety, tolerability and PK of single ascending oral doses (SAD) of CS014; part two explored safety, tolerability, PK, and PD following multiple ascending doses (MAD) of CS014, dosed for seven days. In total, 48 subjects were included in the trial, 30 in the SAD and 18 in the MAD part. The trial was conducted by CTC in Uppsala, Sweden.
Summary of the topline results from the Phase I trial:
- CS014 demonstrated favorable safety and tolerability in healthy volunteers.
- All 48 healthy volunteers completed the study; no early withdrawals or deaths were reported.
- No serious adverse events (SAEs) occurred.
- All treatment-related adverse events (AEs) reported were mild, transient, and fully recovered.
- CS014 achieved levels in the blood stream at and above those projected, based on non-clinical data, to be required for achieving maximal effects on reversal of pulmonary vascular remodeling and fibrosis.
These findings, combined with non-clinical data demonstrating a favorable impact on plexiform lesions in the Sugen/Hypoxia rat model, offer insights that support dose selection and support advancement into Phase II development.
Current status of CS014 development*
The positive Phase I results, combined with strong non-clinical data, supports advancement into Phase II.
Full results from the Phase I trial will be submitted for publication in a peer-reviewed scientific journal.
*Updated August 2025.